In the article, Pragmatic Precision Oncology: The Secondary Uses of Clinical Tumor Molecular Profiling, the authors make the argument that the parsing, categorization, and aggregation of clinical molecular profile data produces increasingly accurate data generation – and the result is a larger repository of genetic variants useful in ongoing research. First published on March 28, 2016, Matthew J Rioth, MD and his co-authors explain how precision oncology increasingly utilizes molecular profiling of tumors to determine treatment decisions with targeted therapeutics.” Dr. Rioth, who also co-authored Implementing and Improving Automated Electronic Tumor Molecular Profiling, reminds us that this trend is moving clinical research in the right direction – resulting in more accurate and reliable data.
The conclusion of the article emphasizes how real-time molecular profiling data is a pragmatic solution to several knowledge management problems in the practice and science of precision oncology.
The benefits described by Dr. Rioth’s research, and the consistency and increased accuracy of their clinical trial outcomes, makes the case for why clinical trial operations should strive for maximum interoperability for long-lasting beneficial outcomes. If you are struggling with integration of clinical study data from multiple systems and platforms, Adaptive Clinical Systems offers a simple, secure, validated, compliant, and cost-effective solution for clinical data integration.
The Adaptive eClinical Bus, a cloud-based hosted service, will integrate with your EDC, ePRO, CTMS, Medical Imaging, 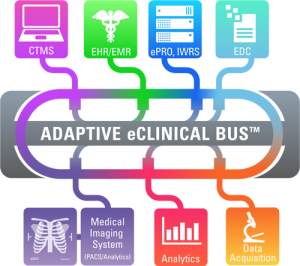 IVR/IWR, and analytical/data visualization systems to ensure accurate and efficient transfer of clinical data for any study of any complexity.
IVR/IWR, and analytical/data visualization systems to ensure accurate and efficient transfer of clinical data for any study of any complexity.
Adaptive Clinical Systems aims to help clinical trial operations in their pursuit of improving clinical trials with improved database efficiencies that lead to better patient treatment. Whether you’re a CRO, Trial Sponsor (Pharmaceutical or Biotechnology Company), Investigator-Initiated Trial, Hospital or Academic Research Center, or a Core Laboratory struggling with integration of clinical study data from multiple systems and platforms, Adaptive Clinical Systems offers a simple, secure, validated, compliant (FDA CFR 21 Part 11 and GxP), and cost-effective solution for clinical data integration.
Want to learn more about Adaptive’s eClinical Bus® Solution?
Click here to Learn more about the Adaptive eClinical Bus®
About Adaptive Clinical
Adaptive Clinical Systems offers a unique, simple, secure, validated, compliant, and cost-effective innovative solution for clinical data integration and interoperability. The cloud-based innovative Adaptive eClinical Bus® solution integrates clinical study data from multiple systems and platforms — EDC, eCOA, CTMS, Medical Imaging, IRT, analytical/data visualization systems and others — to ensure accurate and efficient transfer of clinical data for any study of any complexity while going well beyond simple and difficult to scale integration to full, real-time interoperability.
The award-winning Adaptive eClinical Bus software includes “connectors” for many leading clinical trial software tools from well-known vendors such as Omnicomm, Medidata, BioClinica, and Clinical Conductor to open source clinical trial tools such as OpenClinica and Clinovo. Connectors can also leverage internally-developed and proprietary systems and help customers retain their competitive edge. Adaptive Clinical’s eClinical Bus® can easily integrate technology into an interoperable, efficient, and accurate clinical trials system that streamlines processes and improves data reliability and offers the freedom to choose the best eClinical tools of any third-party or proprietary systems while enjoying all the benefits of a fully integrated system.
To learn how Adaptive Clinical Systems can help improve the interoperability of your clinical trials, click here.
Interested in Pragmatic Precision Oncology: The Secondary Uses of Clinical Tumor Molecular Profiling?
Want to read the full article by , , , ,
